The Schrödinger Equation: The Mathematics of Quantum Waves
The Schrödinger Equation: The Mathematics of Quantum Waves
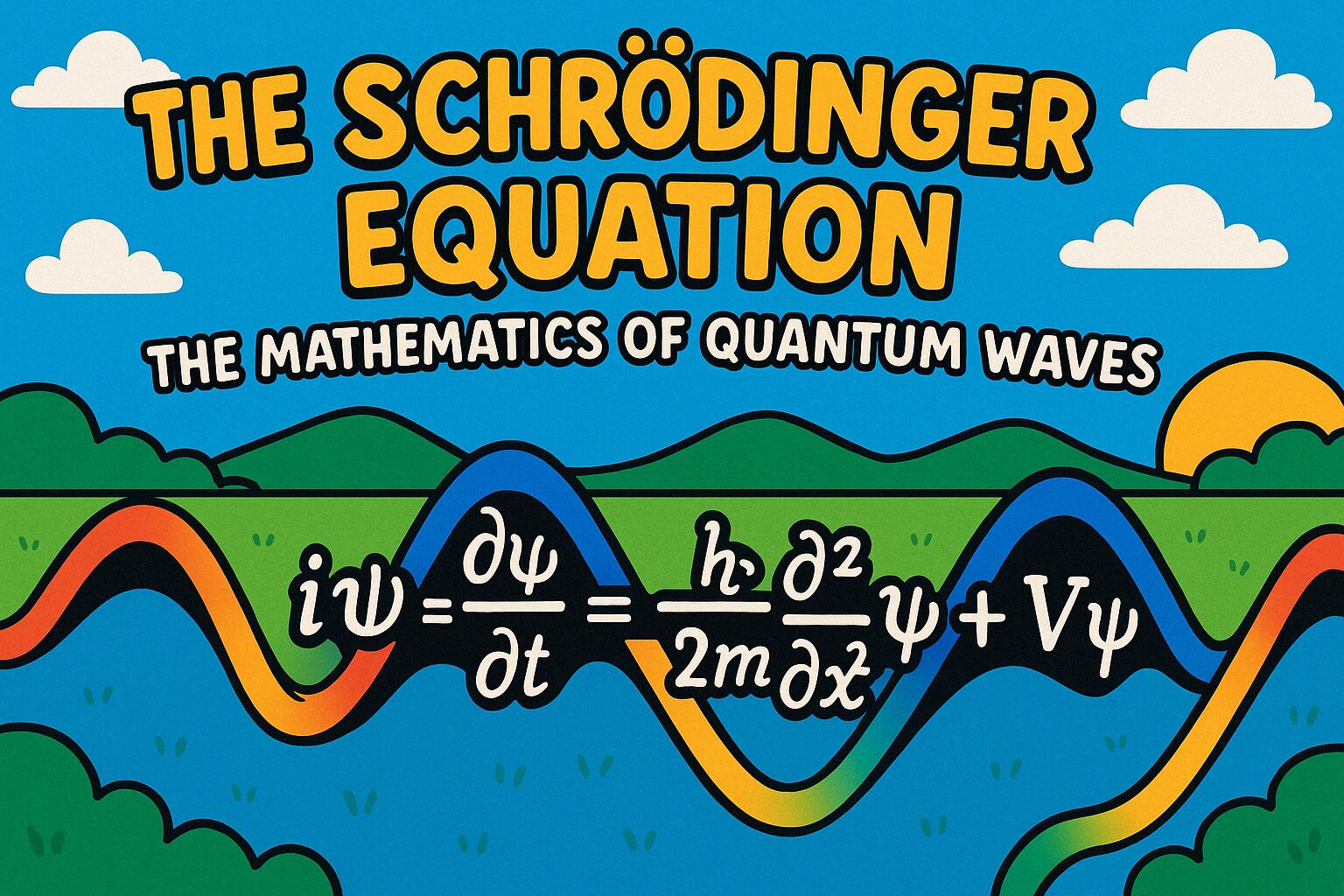
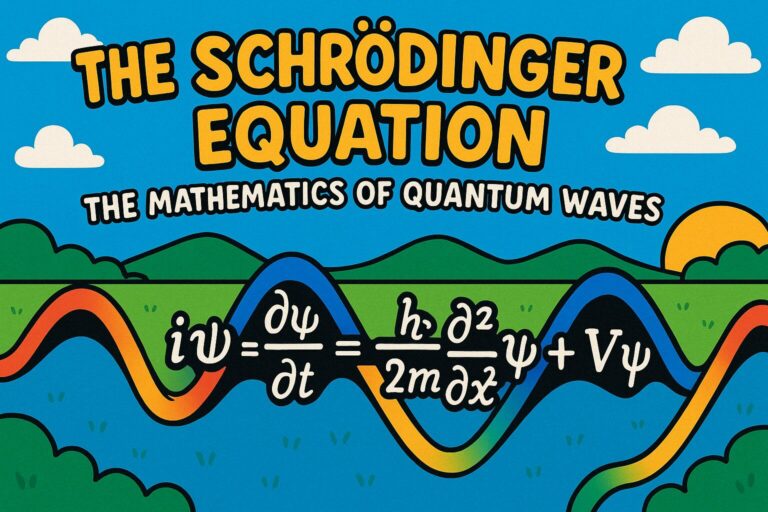
The Schrödinger Equation is one of the most important ideas in modern physics. It explains how tiny particles like electrons behave like waves, which is a key part of quantum mechanics. This wave-like behaviour is described by something called a wavefunction. Scientists use this quantum wave equation to predict where particles might be and how they move over time.
Before this equation came along, physics was mostly about solid, predictable rules like Newton’s laws. But once people started exploring atoms and subatomic particles, they noticed things didn’t always follow those old rules. That’s where quantum mechanics comes in – it helps explain weird things like particles being in more than one place at once.
The Schrödinger Equation gives us a mathematical way to describe those strange behaviours. You might think of it like a recipe that shows how quantum waves change. And just like a music note has a shape and rhythm, so does a particle’s wavefunction. In this topic, we’ll explore how the equation works, what it means, and why it’s such a big deal in physics.
This topic is part of our Info Zone collection. You can read the full topic, once logged in, here: The Schrödinger Equation: The Mathematics of Quantum Waves
You’ll also find a full Lesson Plan and a handy Parent Q & A sheet, for this topic, ready to use..
Members Only
You need to be registered and logged in to access this learning resource and other member only content. It only costs £1.99